Selecting your brazing materials.
Before choosing a filler metal, you must understand and evaluate the three basic characteristics of fiIIer metals: physical properties melting behavior and forms available. Let’s look at each of these characteristics.
Physical properties and melting behavior.
The physical properties of a filler metal are based on metallurgical composition. (Brazing filler metals are invariably alloys, made of two or more “pure” metals.) This composition determines whether the filler metal is compatible with the metals being joined – capable of wetting them and flowing completely through the joint area without forming detrimental metallurgical compounds. Plus, special service or production requirements may call for special properties. For example, if you’re brazing in a vacuum, you need a filler metal free of any volatile elements, such as cadmium or zinc. Some electronic components require filler metals of very high purity. And corrosion-resistant joints need filler metals that are both corrosion- resistant and compatible with the base metals being joined. Melting behavior is also based on metallurgical composition. Since most filler metals are alloys, they usually do not melt the same as pure metals which change from a solid to a liquid state at one temperature. However, there is an important exception to this statement. There is a class of alloys, termed “eutectics,” that do melt in the same manner as pure metals. An example of an eutectic composition is Handy 8 Harman’s Braze 721, a simple silver-copper alloy made of 72% silver and 28% copper. This filler metal melts completely at a single temperature – 1435°F (780°C). In metallurgical terms, its melting point (solidus) and flow point (liquidus) are identical. This melting behavior is shown on the following chart. Note that at the 72% silver, 28% copper composition, liquidus and solidus temperatures are the same.
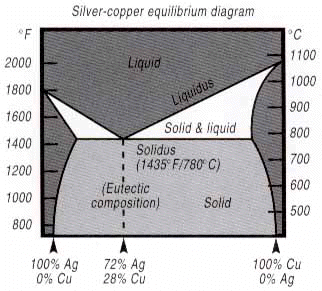
And, the alloys to the left or right of this eutectic composition do not go directly from a solid to a liquid state, but pass through a “mushy” range where the alloy is both solid and liquid. This range is the difference between the “solidus” temperature, which is the highest temperature at which the alloy is completely solid (i.e., the point where melting starts when the alloy is heated) and the “liquidus” temperature, which is the lowest temperature at which the alloy is completely liquid (i.e., the point where solidifying starts as the alloy is cooled.)
Importance of “melting range.”
Look at a couple of examples. If you are brazing an assembly with a narrow, closely controlled clearance, Handy & Harman’s Braze 560 filler metal works weII. This cadmium free alloy begins to melt at 1145°F/620°C and flows freely at 1205°F/650°C. Its melting range is 60°F/ 15°C. When brazing an assembly with wide clearances (greater than .005), select a filler metal like the cadmium free Braze 380. As it starts to melt at I 200°F/650°C and becomes fully liquid at 1330°F/720°C, its flow characteristics are sluggish enough to fill wide gaps.
Consider the “liquidus temperature.”
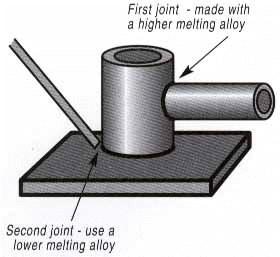
In all brazing applications, the “liquidus temperature” of the brazing filler metal is a critical factor. Since in brazing you never want – or need – to melt the base metals, you should select a filler metaI whose liquidus temperature is lower than the solidus temperature of both of the base metals being joined. There are several brazing situations in which the liquidus temperature factor calls for special consideration. For example, when “step brazing” an assembly – that is, brazing in the vicinity of a previously brazed joint, you don’t want the second brazing operation to disturb the first joint. The way to prevent this is to use more than one type of filler metal. Make the second joint with a filler metal lower in liquidus temperature than that used for the first joint. This way you are assured the first joint will not be re- melted when making the second. Also consider liquidus temperature when brazing assemblies that must be heat treated. In these instances, you have two options. You can heat treat and then braze – in which case you should select a filler metal whose liquidus temperature is lower than the heat-treating temperature. This way the hardness properties won’t be adversely affected by brazing. Or you can heat treat and braze simultaneously. In this case, the liquidus temperature of the filler metal should be closely equivalent to the heat treating temperatures.


Follow Us!